CE Marking is a ‘mark of conformity’ or ‘mark of compliance’ which states that a particular product meets requirements of European Union legislation. It is a mandatory mark to place / sell a product in Europe. CE Marking is a kind of guarantee for consumers that “the product is safe for use”. CE Marking is also considered as most recognized product certification in world. CE marking is the manufacturer’s declaration that the product meets the requirements of the applicable EC directives.
Why CE Marking is required?
- CE Mark is a mandatory to market your products in Europe
- CE Mark ensures that product is safe for human use
- CE Marked products can be circulated freely in 30 EEA countries
- It has become a requirement in global market
- It is required in Indian government tenders also
- CE Marking increases image of product in market
- Big buyers also prefer to buy CE Marked products
- CE products are treated in EEA without restrictions
The CE Marking is required only for the following types of products:
- Toys
- Machinery
- Electrical equipment
- Personal protective equipment
- Pressure equipment
- Medical devices
- Active implantable medical devices
- In vitro diagnostics
- Radio and Telecommunications terminal equipment
- Simple pressure vessels
- Gas appliances
- Lifts
- Recreational craft
- Equipment and protective systems for use in explosive atmospheres
- Non-automatic weighing instruments
- Cableways
- Construction products
- Explosives for civil use
- New hot water boilers
Implementation Plan:
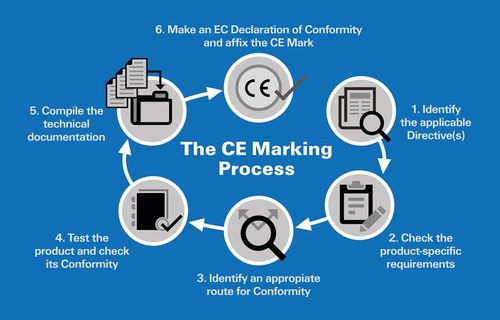
Seven Steps of CE-Marking
Step 1: The first steps towards CE-marking your product is determining your product’s application and establishing which directives apply to it.
Step 2: Applying standards
The process is getting your product to meet the essential requirements laid down in the directives.
Step 3: Make sure your product is safe and be able to prove it. So carry out a risk assessment or analysis and show how you meet the requirements laid down in the directives.
Step 4: Technical document
Drawing up technical documentation or a technical file, intended to provide information on the design, manufacture and operation of your product.
Step 5: User Manual
The fifth step is to apply the product with instructions for the end user, including legal obligations, instructions for use and clear documentations, stating the purpose of the product and the risks related to its use under normal circumstances.
Step 6: Declaration of conformity
The sixth step is the declaration on conformity, one of the final steps in the CE marking procedure. The declaration of conformity must contain all relevant product information, including which EU directive(s) the product now complies with.
Step 7: Affixing the CE mark
The seventh step is to affixing the CE-mark, which means you declare to all parties Involved that your product conforms to all applicable provisions.
